Simple microgels for complex problems.
Our experience working with our industry collaborators have given us an insight into what is achievable with the Progel encapsulation technology with its core application being in food, pharmaceuticals and nutraceuticals.
-
 Gastric Protection
Gastric Protection -
 Controlled Intestinal Release
Controlled Intestinal Release -
 Enhanced Probiotic Viability
Enhanced Probiotic Viability -
 Stabilization
Stabilization -
 Taste Masking
Taste Masking
Taste Masking
Protein, Plant Extract, Pharmaceuticals & Chemicals
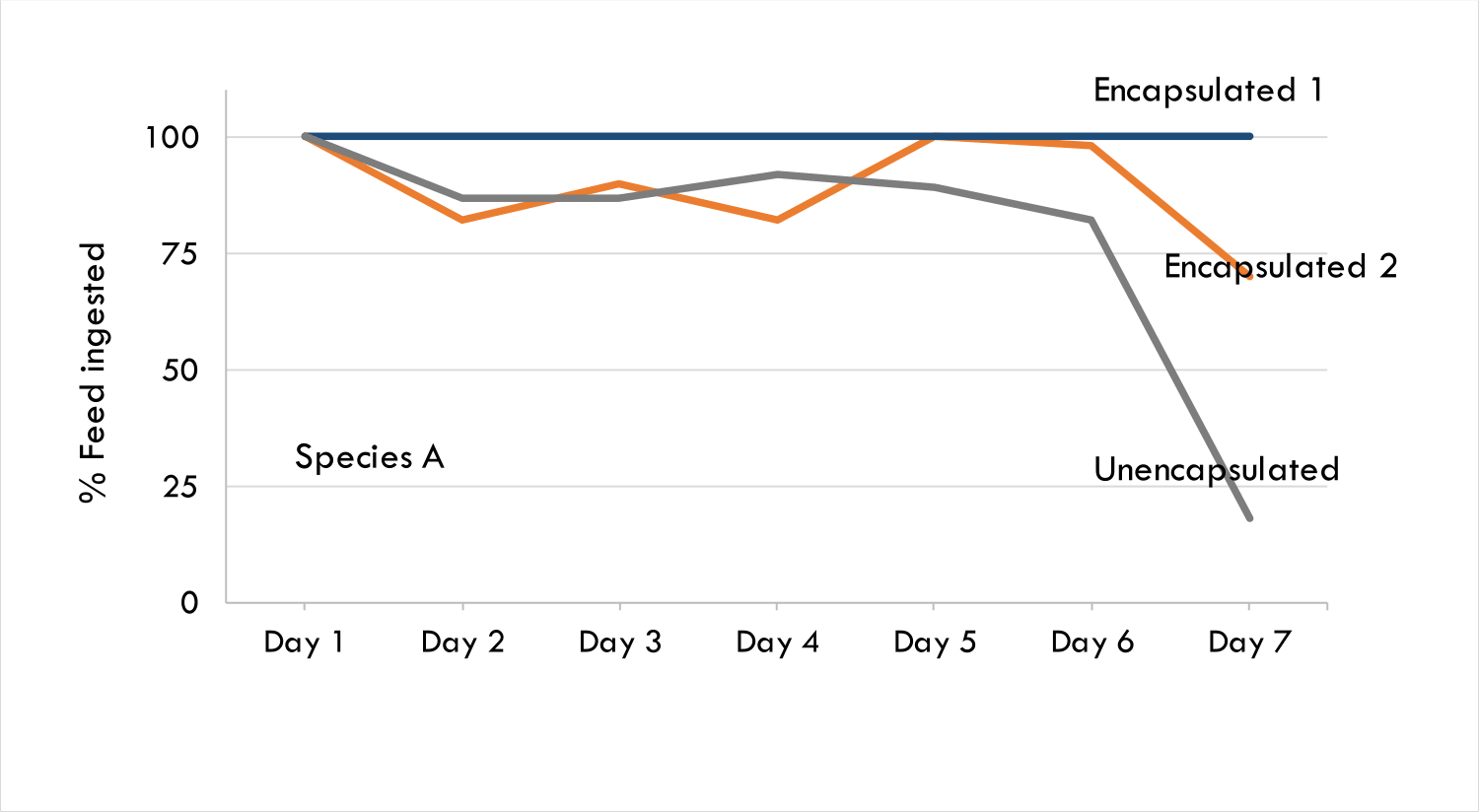
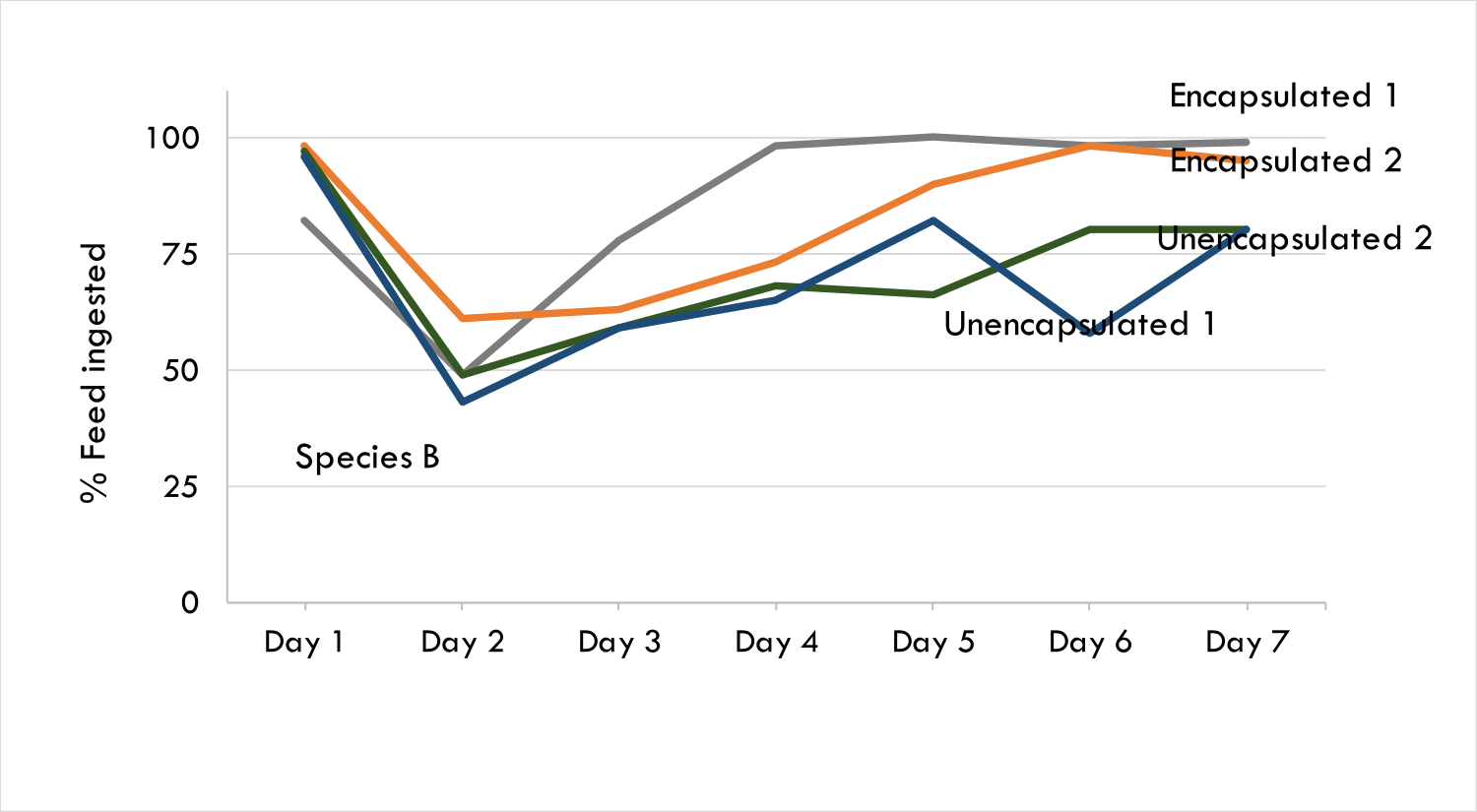
Taste masking increases the palatability of bitter actives such as pharmaceuticals and polyphenols. The Progel technology has been proven in animal models using Bitter Drug A, which is usually administered in animal feed. Bitter Drug A was encapsulated in Progel’s alginate microgels with farm trials being conducted across two continents using two animal species. Encapsulated Bitter Drug A was preferred by both species of farm animals and through sera analysis it was shown that encapsulated Bitter drug A was released upon ingestion, evidenced by the presence of active that was detected in the animal tissue.
Stabilization
Lipids, Lipophilic Actives & Pharmaceitucals
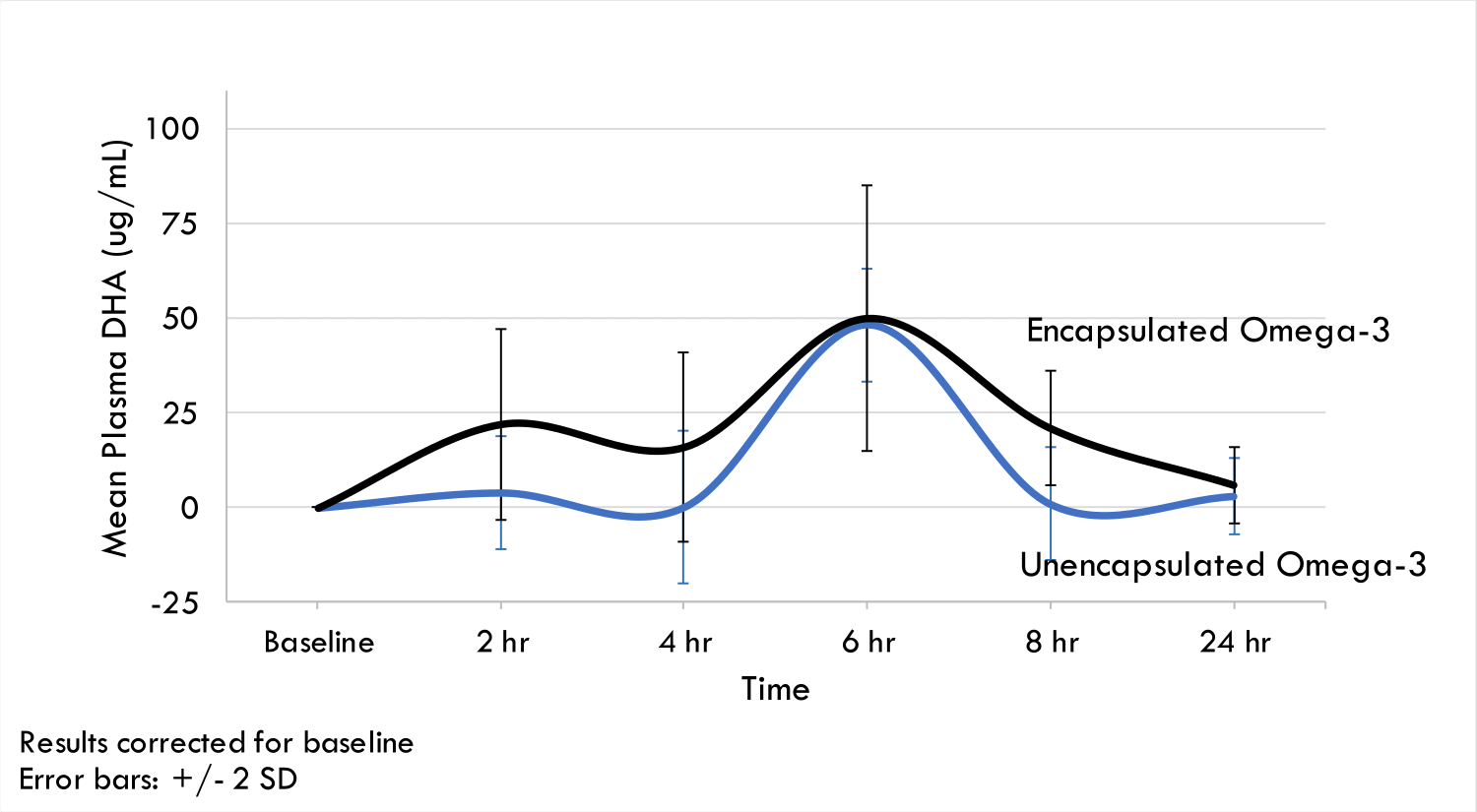
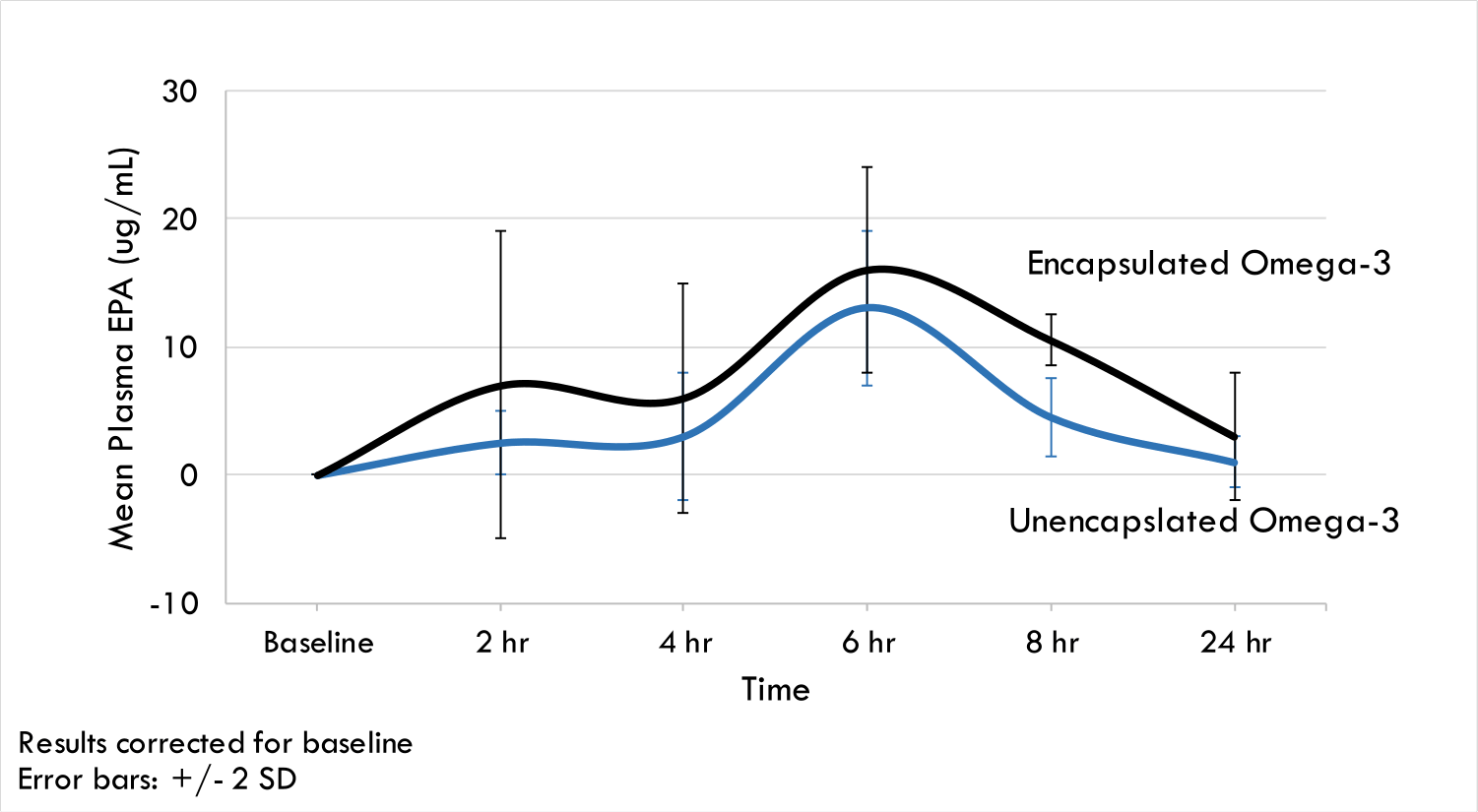
Encapsulation helps stabilize lipid emulsions against physical and chemical instabilities and at the same time, increases bioavailability. A crossover clinical trial conducted with healthy adults showed that DHA and EPA absorption, as measured in blood plasma, was higher (by 50%) in individuals fed encapsulated Omega-3. It is suggested that the encapsulated lipids are able to transit through the stomach intact and that the mucoadhesive microgels adhere to the mucosa in the intestines where they release the lipid nanoemulsion for rapid absorption.
Gastric Protection
Proteins, Plant Extracts, Enzymes & Probiotics
Protecting an active during the transition through the stomach is crucial to maximise absorption in the lower intestines. The highly acidic environment coupled with presence of digestive enzymes act together to deactivate actives. Not only are protected actives able to retain their structure (proteins) or activity (enzymes), they are also delivered intact to the intestine. Bioavailability increases and the maximum health benefit is delivered.
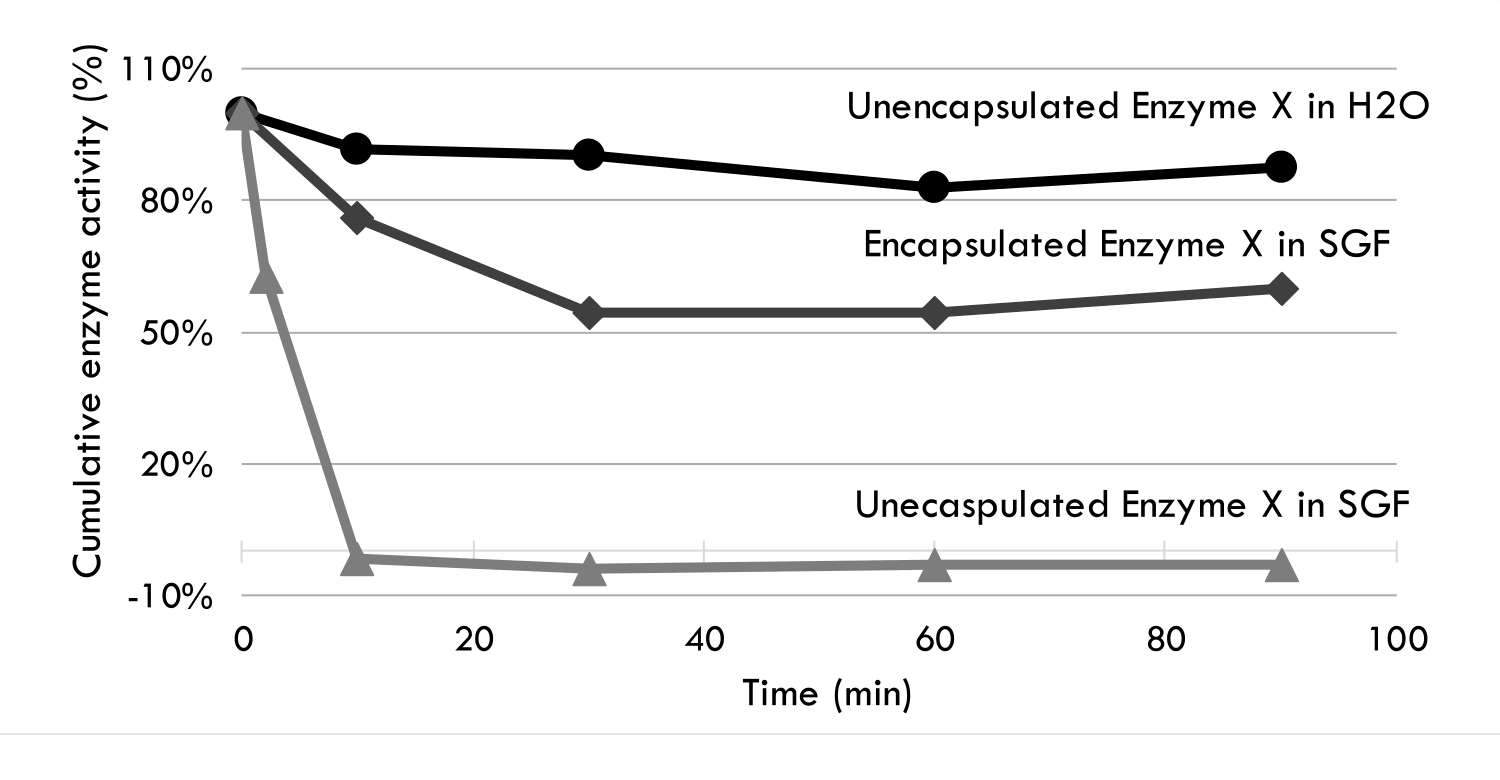
Enzyme X is used to treat ailments in young farm animals. In adult farm animals, Enzyme X is not as effective due to the low pH environment and enzymes present in the fully developed stomach. In vitro studies show that encapsulation protects Enzyme X in simulated gastric environment which suggests that Enzyme X will be able to retain its activity during its transition through the stomach. This opens up potential treatment for ailments in adult animals and humans.
Encapsulation protects bovine lactoferrin against digestive enzymes in the stomach. In in vitro studies, unencapsulated lactoferrin is quickly digested by pepsin while most of the encapsulated lactoferrin are intact. Human trials showed that encapsulated lactoferrin supports a healthy immune and digestive system.
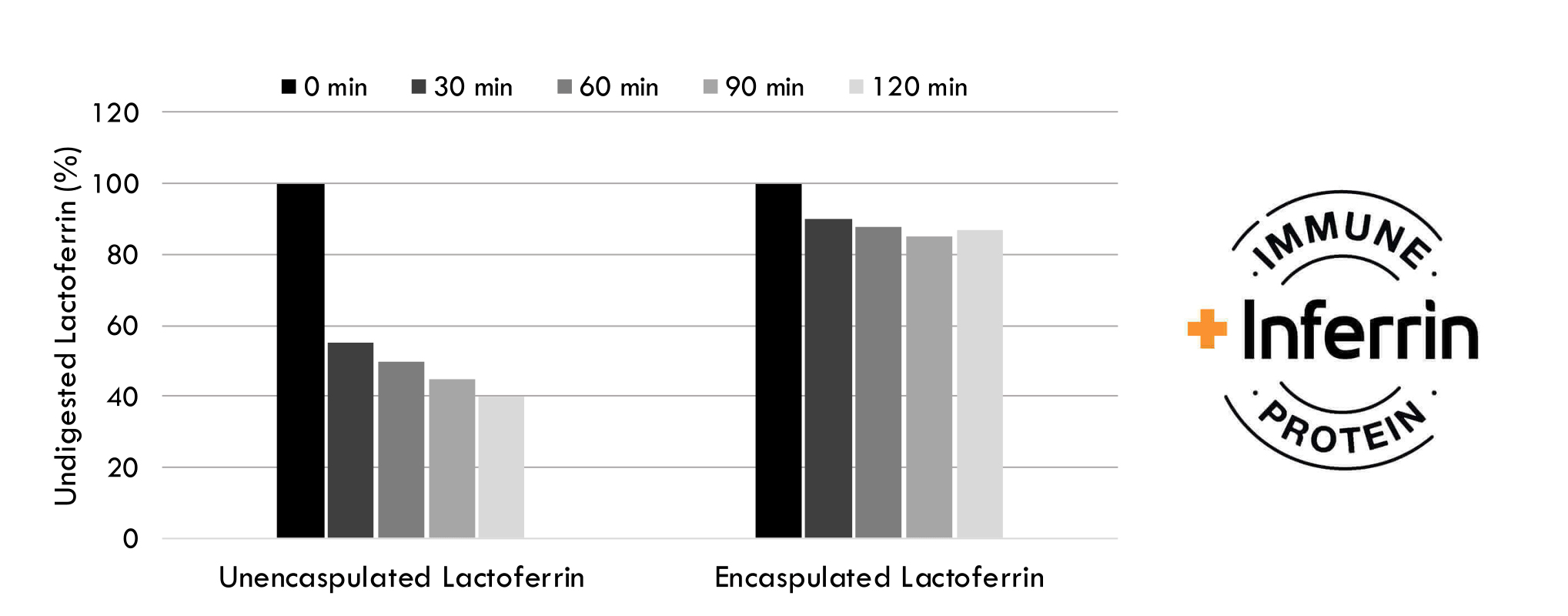
Enhanced Probiotic Viability
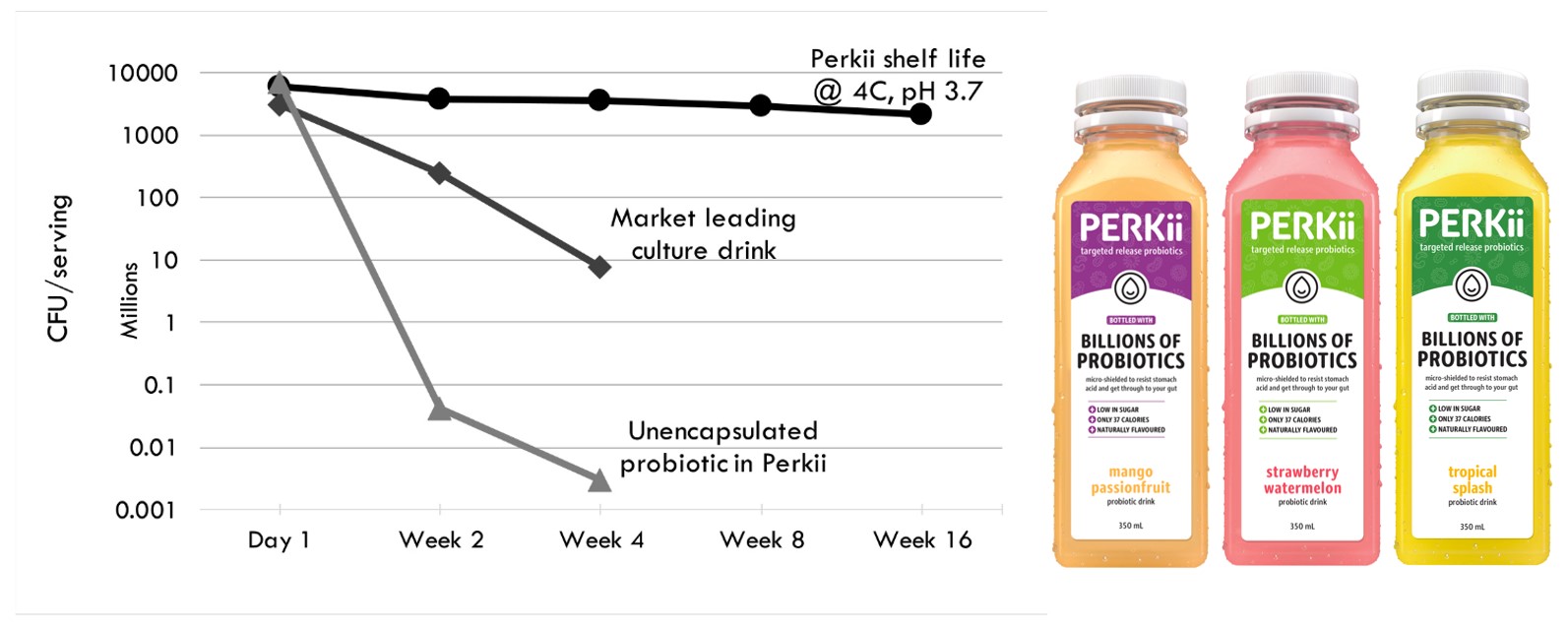
Probiotics are fastidious organisms that are sensitive to their environment. Encapsulation significantly improves the viability of probiotics in both acidic dairy and non-dairy food products such as yoghurt, protein drinks, milks and fruit juices over the product shelf life. Furthermore, in-vitro experiments show that encapsulation improves the viability of probiotics in simulated stomach conditions, allowing targeted release into the gut..
Progel encapsulated probiotics has been proven in food products. Launched in 2016, PERKii utilises this technology in its probiotic beverages to shield the Lactobacillus Casei and Bifidobacterium strains, prolonging their survivability in the drink and enabling the targeted release of these microorganisms in the gut. The micron sized microgels prevent fermentation and are tasteless, allowing PERKii to deliver these beneficial live organisms in a fruit-flavoured, non-dairy, lactose, gluten and added sugar free carrier.
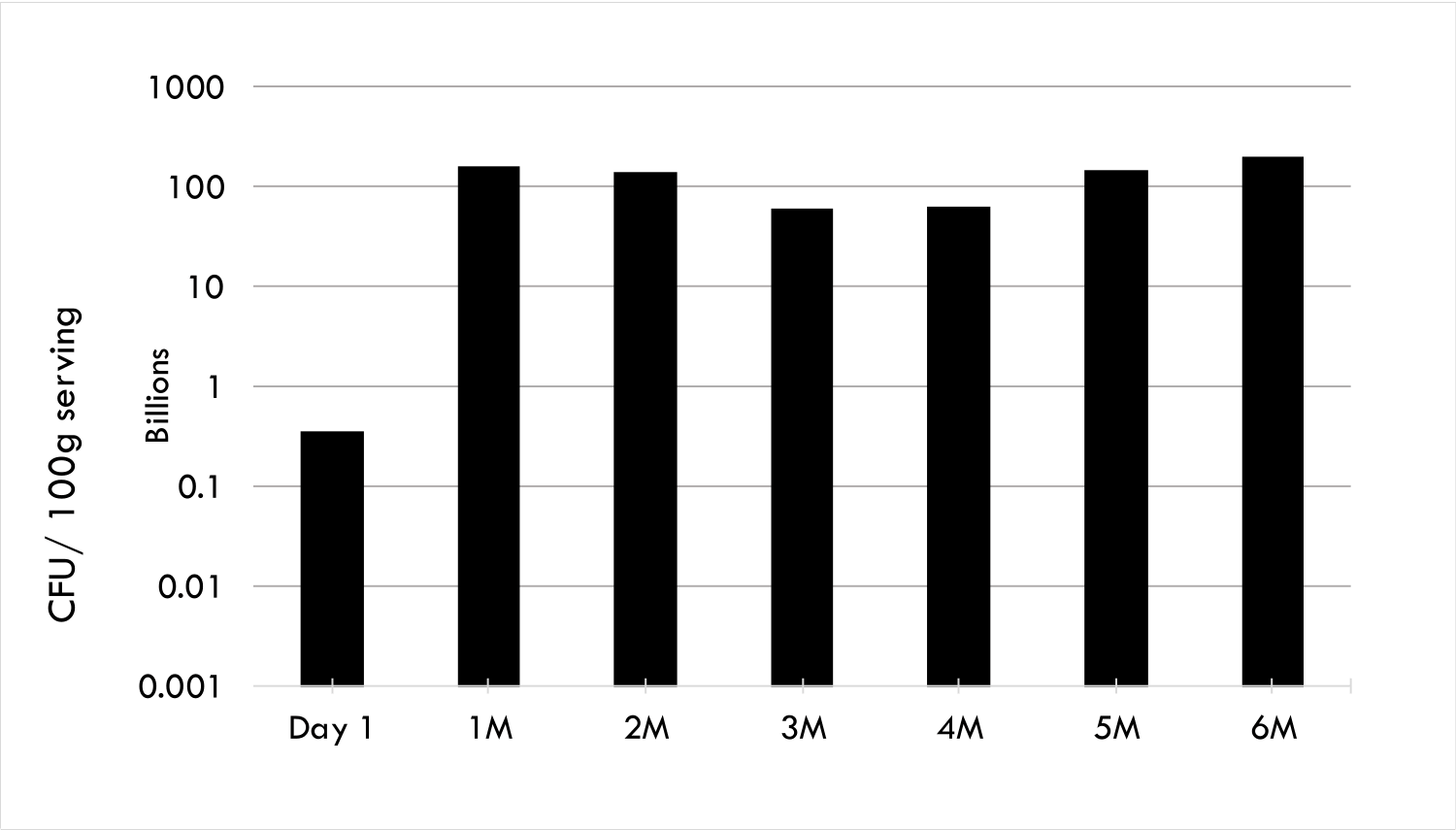
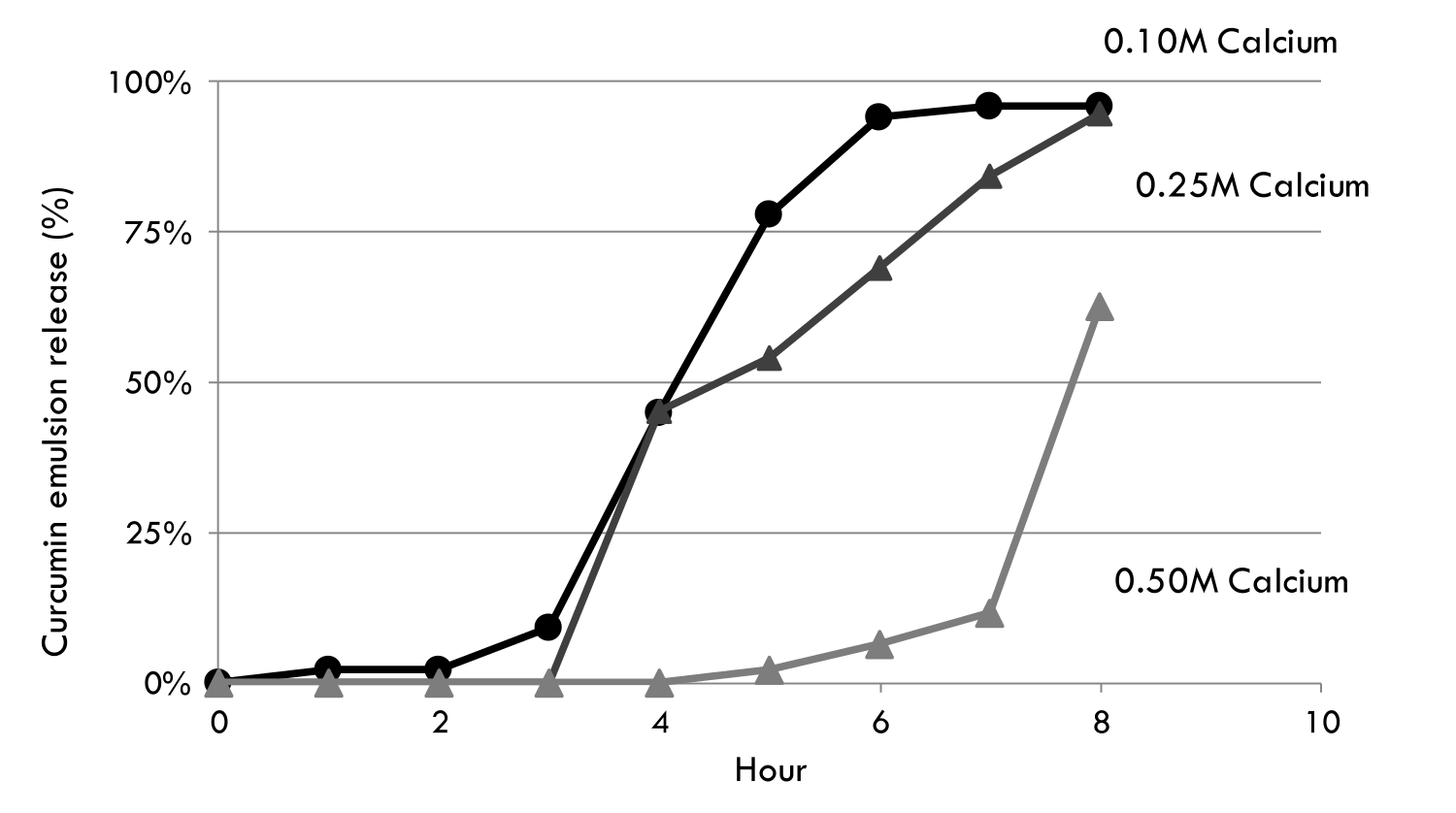
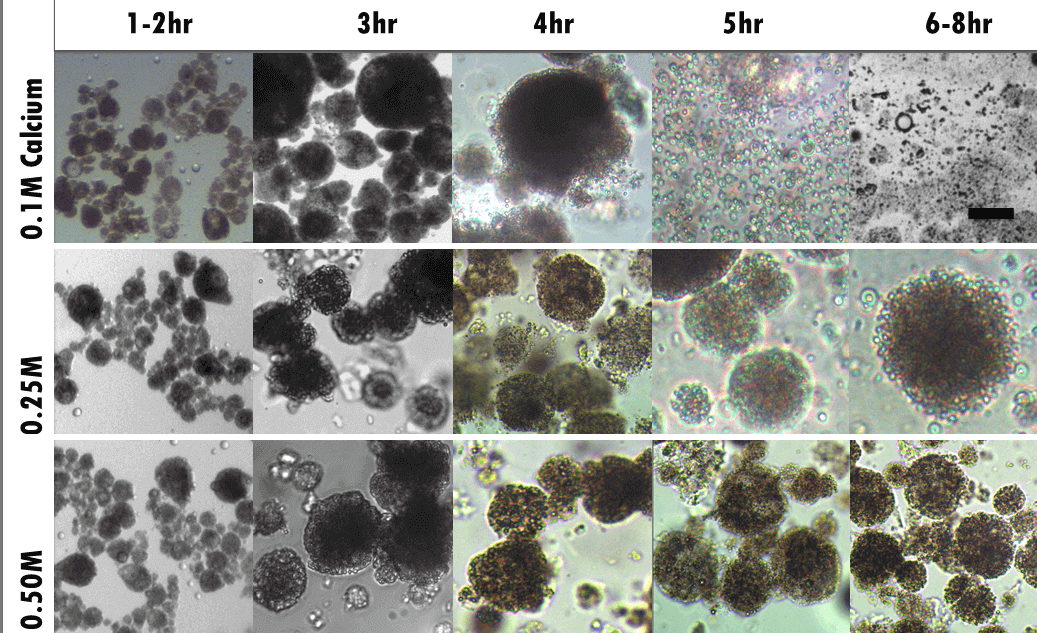
Controlled Intestinal Release
Proteins, Plant Extracts, Enzymes, Lipids, Pharmaceuticals & Probiotics
In response to elevated pH conditions, similar to intestinal conditions, the alginate microgels swells and starts to disintegrate. This initiates the release of active from within the microgels. How quickly the contents are released can be modulated by the concentration of calcium used to gel the microgels. The concentration of calcium used to gel the alginate gel is proportional to gel strength and cross-linking density. Release of the contents is rapid when a low concentration of calcium is used but delayed when a higher calcium concentration is used.